11.03.2025
New Paper on a general approach for activity-based protein profiling of oxidoreductases with redox-differentiated diarylhalonium warheads
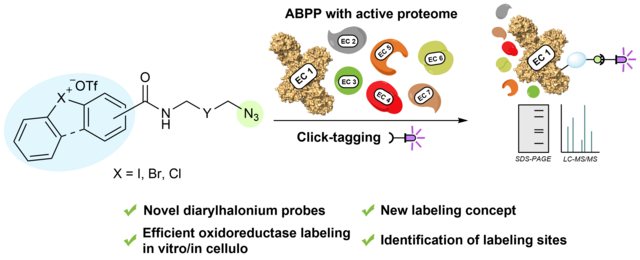
In our latest study, published in Chemical Science, we describe the
development of ABPP probes for the simultaneous labeling of various subclasses
of oxidoreductases. The probe warheads are based on hypervalent diarylhalonium
salts, which show unique reactivity as their activation proceeds via a reductive
mechanism resulting in aryl radicals leading to covalent labeling of liver
proteins at several different amino acids in close proximity to the active
sites. The redox potential of the probes can be tuned by isosteric replacement
varying the halonium central atom. ABPP experiments with liver using 16 probes
differing in warhead, linker, and structure revealed distinct overlapping
profiles and broad substrate specificities of several probes. With their
capability of multi oxidoreductase subclass labeling – including rare examples
for the class of reductases – and their unique design, the herein reported
probes offer new opportunities for the investigation of the “oxidoreductome” of
microorganisms, plants, animal and human tissues.
https://pubs.rsc.org/en/content/articlelanding/2025/sc/d4sc08454c
07.02.2025
New Paper on One-Pot Hetero-Di-C-Glycosylation of the Natural Polyphenol
Phloretin by a Single C-Glycosyltransferase
In this collaboration with the Institute of Biotechnology and Biochemical
Engineering at the Graz University of Technology, published in Biotechnology &
Bioengineering, an efficient system for synthesizing high-value polyphenol
hetero-di-C-glycosides with two distinct C-glycosyl residues was established
using a two-step glycosylation catalyzed by C-glycosyltransferase in one pot.
The desired C-β-galactosyl-C-β-glucosyl and C-β-glucosyl-C-β-xylosyl
phloretin were obtained at concentrations of 0.6 and 6 g/L, respectively, with
quantitative conversion. The in-situ formation of UDP-xylose from UDP-
glucuronic acid via UDP-xylose synthase, along with the regeneration of UDP-
glucose through sucrose synthase, was carried out during the cascade synthesis
of C-β-glucosyl-C-β-xylosyl phloretin.
https://doi.org/10.1002/bit.28948

17.12.2024
Congratulations to Leo Krammer
Leo Krammer just defended his PhD thesis on “Synthesis and Evaluation of Redox-
Active Small Molecule Probes for their Use in Activity-Based Proteomics". We
want to thank him for the last years in which he became an essential member of
the Institute and congratulate on doing a marvellous job at his defense!
29.08.2024
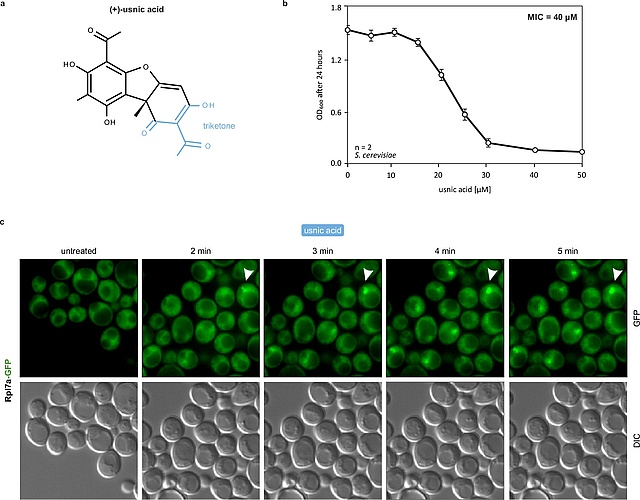
New paper on a novel ribosome biogenesis inhibitor blocking nucleolar pre-60S
maturation
In our latest collaboration, published in Nature Communications, we demonstrate that usnic acid, a lichen secondary metabolite, inhibits the maturation of the large ribosomal subunit in yeast.By combining biochemical characterization of pre-ribosomal particles with a quantitative single-particle cryo-EM approach, changes in nucleolar particle populations upon drug treatment were monitored. We show that usnic acid rapidly blocks the transition from nucleolar state B to C of Nsa1-associated pre-ribosomes, depleting key maturation factors such as Dbp10 and hindering pre-rRNA processing. This primary nucleolar block rapidly rebounds on earlier stages of the pathway which highlights the regulatory linkages between different steps. This in-depth characterization of the effect of usnic acid on ribosome biogenesis may have implications for its reported anti-cancer activities.
https://www.nature.com/articles/s41467-024-51754-3
28.06.2024
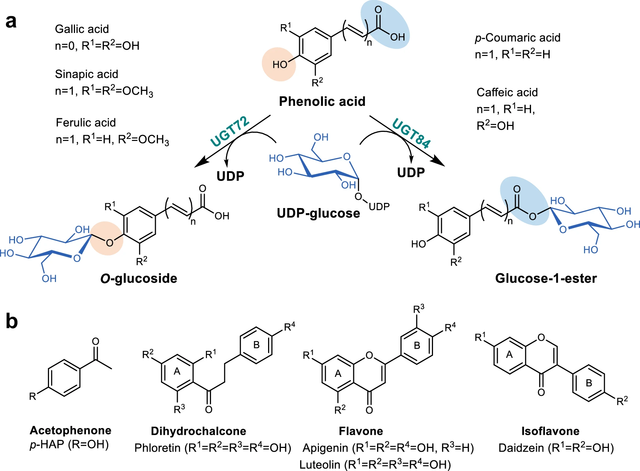
New Paper onDiscovery, Characterization, and Comparative Analysis of New UGT72
and UGT84 Family Glycosyltransferases
In this collaboration with the Institute of Biotechnology and Biochemical
Engineering at the Graz University of Technology, published in Communications
Chemistry, the glycosylation capabilities and substrate preferences of newly
identified plant uridine diphosphate (UDP)-dependent glycosyltransferases (UGTs)
within the UGT72 and UGT84 families is explored.Through detailed time course and
reaction rate analyses combined with product isolations and characterizations,
distinct regio-selectivities and specific activities are revealed towards a
variety of polyphenolic substrates.The findings hold the potential to enhance
future biotechnological applications, including the design and engineering of
UGTs for specific glycosylation reactions.
https://www.nature.com/articles/s42004-024-01231-1
24.04.2024
New paper on Ene-Reductase Catalyzed Asymmetric Synthesis of Chiral 2-Cyclohexenones
In our latest pubication, published in ACS Catalysis, wedescribe the use of ene-reductases OPR3 and YqjM for the efficient asymmetric synthesis of chiral 4,4-disubstituted 2-cyclohexenones via desymmetrizing hydrogenation of prochiral 4,4-disubstituted 2,5-cyclohexadienones. This transformation breaks the symmetry of the cyclohexadienone substrates, generating valuable quaternary stereocenters with high enantioselectivities (up to >99% ee). In addition, the synthetic potential of the resulting chiral enones was demonstrated in several diversification reactions in which the stereochemical integrity of the quaternary stereocenter could be preserved.
https://pubs.acs.org/doi/full/10.1021/acscatal.4c00276

01.03.2024
Congratulations to Michael Frieß
Today Michael Frieß successfully defended his PhD thesis on "Investigation of Non-natural Ene-reductase Catalyzed Reactions". We want to congratulate Michael on this great achievement and thank him very much for all the years as a great colleague to all of us at our institut

24.01.2024
New Paper on Cofactor Specificity in the FMN-dependent Family of Ene-reductases
In this collaboration, published in The FEBS Journal, we postulate that cofactor specificity is determined by the arginine triad/loop 6 and the residue(s) controlling access to a hydrophobic cleft formed by the β-hairpin flap. Our results suggest that in order to switch the coenzyme preference in NADPH-dependent OYEs, the β-hairpin flap region needs to be targeted by structure-based engineering approaches to create a high-affinitybinding site for NADH, for example, by replacing L140 for an adenine ring-stabilizing phenylalanine andadjusting the length of the hairpin so that it resembles more that of NADH-preferring ERs. Conversely, loop 6 should be the engineering target to switch the nico-tinamide affinity in NADH-dependent OYEs. Generation of such SlOPR3 variants with switched coenzyme preference is currently underway.

06.01.2024
New Paper on Design, Synthesis and Catalytic Application of Water-soluble 1,1′-Bis(diphenylphosphino)ferrocene Ligands
In our latest study, published in Molecular Catalysis, we disclose synthetic strategies towards the first hydrophilic dppf derivatives overcoming issues typically encountered due to their intrinsic oxidizability. The novel ligands were spectroscopically characterized and showed their catalytic potential in the Pd-catalyzed Tsuji-Trost allylation of amines in water furnishing pharmaceutically relevant N-allylated products (e.g., naftifine, cinnarizine) through a scalable, safe and environmentally benign process.

21.11.2023
New Review on Recent Advances in Pd-Catalyzed Suzuki-Miyaura Cross-Coupling
Reactions with Triflates or Nonaflates
Pd-catalyzed cross-coupling between aryl- and alkenyl halides and nucleophilic organometallic substrates, such as organoboronic acids (Suzuki-coupling), organostannanes (Stille coupling), or zinc organyls (Negishi coupling) has become one of the most widely used C−C coupling reactions, which has found many applications in medicinal chemistry and material sciences. In our latest review, published in Advanced Synthesis & Catalysis, we aim to systematically review the developments in the Suzuki coupling of organotriflates and nonaflates over the last decade, with a focus on new catalysts and strategies to overcome the described side reactions. Additionally, we provide a matrix table of substrate combinations with reaction conditions, which should enable the practitioners to efficiently find conditions that could be applied for their planned syntheses.

14.05.2023
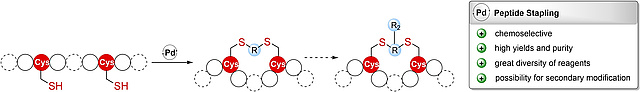
New paper on Constraining and Modifying Peptides Using Pd-Mediated Cysteine
Allylation
In this collaboration, published in ChemBioChem, with research groups from the
University of Vienna an extension of the recently introduced chemoselective
Pd-catalyzed cysteine allylation reaction to peptide stapling and further
modification of the resulting alkene-containing staples to introduce additional
probes into such stabilized peptides is reported.
14.02.2023
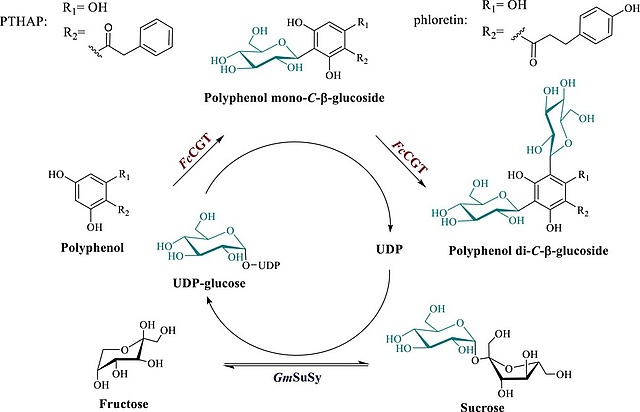
New Paper on Biocatalytic Production of Polyphenolic Natural Product Di-C-β-glucosides
In this collaboration with the Institute of Biotechnology and Biochemical Engineering at the Graz University of Technology, published in Biotechnology & Bioengineering, a biocatalytic process technology for reaction-intensified production of the di-C-β-glucosides of two representative phenol substrates (phloretin and phenyl-trihydroxyacetophenone) from sucrose is reported. Collectively, this study demonstrates efficient glycosyltransferase cascade reactions for flexible use in natural product di-C-β-glucoside synthesis from expedient substrates.
18.01.2023
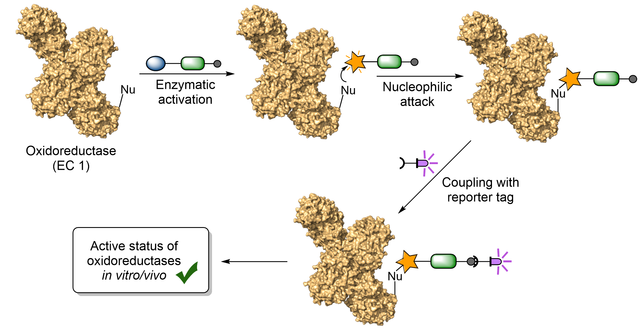
New review on activity-based protein profiling of oxidases and reductases
Activity-based protein profiling (ABPP) as a proteomic tool allows measuring the
activity of enzyme classes or distinct proteins in their cellular context,
annotating the function of uncharacterized proteins, and investigating the
target profile of small molecule inhibitors. In our latest review, published in
the Israel Journal of Chemistry, we provide an update to our previous review,
focussing on the recent advances of ABPP of oxidases and also reductases. This
article will be part of a special issue dedicated to Prof. Benjamin F. Cravatt,
the pioneer of ABPP and recipient of the 2022 Wolf Prize in Chemistry.
https://onlinelibrary.wiley.com/doi/full/10.1002/ijch.202200086
11.11.2022
Loss of Prof. Dr. Klempier
The Institute of Organic Chemistry mourns the loss of ao.Univ. Prof. Dr. Norbert Klempier (born 7.11.1953), who passed away after enduring a long fight against cancer on 11.11.2022. Prof. Klempier studied Chemistry at the University of Graz receiving his PhD in 1981. After post-doctoral research at Northeastern University in Boston/USA and work in industry, he joined the Institute of Organic Chemistry at TU Graz in 1988. Prof. Klempier established a state-of-the-art chiral chromatography lab at our institute and was an early pioneer in biocatalysis research investigating the synthetic potential of hydroxynitrilases, nitrile hydratases and nitrile reductases. Over many years, Prof. Klempier was organizing the Undergraduate Organic Chemistry lab for BSc Chemistry students, where he inspired students by his passion for chemistry and his great empathy and fairness. We will remember Prof. Klempier as a great scientist and a very fine colleague

27.09.2022
Congratulations to Christian Lembacher-Fadum
Today Christian Lembacher-Fadum defended his PhD thesis on "Synthesis of
N-Heterocyclic Inhibitors and Tool Compounds for Drug Discovery and Chemical
Biology". We want to congratulate Christian on this fantastic achievement, as he
has also been working in industry since december 2021 while writing his thesis,
and thank him very much for all the years as a great colleague to all of us at
our institute!

14.07.2022
Sub Auspiciis Doctoral Graduation of Dr. Thomas Schlatzer
Today, our group alumni Dr. Thomas Schlatzer received his promotio sub
auspiciis Praesidentis rei publicae, which is the highest possible distinction
for academic achievements for a doctoral degree in Austria. In this solemn
event, Thomas was awarded with his doctoral degree and a ring of honour by
president Dr. Alexander van der Bellen.
Thomas is currently working as a postdoctoral researcher in the group of Prof.
Veronique Gouverneur at the University of Oxford and we wish him all the best
for his future career!

14.05.2022
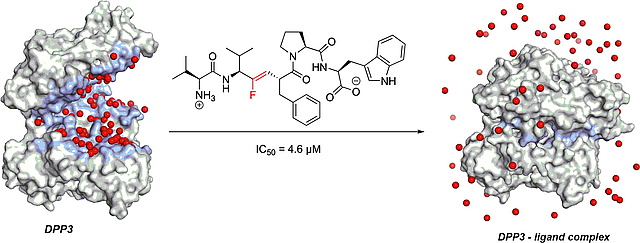
New paper on fluororethylene-peptidomimetic inhibitors of DPP3
In this study, published in Bioorganic & Medicinal Chemistry, we describe the
design and synthesis of the so far most effective peptide-based inhibtor of
dipeptidyl peptidase III (DPP3). Starting from the slowly converted peptide
substrate tynorphin and by replacing the scissile bond with a fluoroethylene
bioisostere, thenewly reported ground state inhibitor outcompetes the previously
reported transition state mimetic inhibitor HER.
01.04.2022
New Paper on Small-Molecule Inhibitors Targeting Lipolysis in Human Adipocytes
In our latest collaboration with the Institute of Molecular Biosciences at the
University of Graz, published in Journal of the American Chemical Society, we
reportthe development and biological characterization of the first
small-molecule inhibitor of human Adipose Triglyceride Lipase (ATGL), which is a
key enzyme in lipolysis. Detailed insights into the enzyme–inhibitor
interactions could be revealed through combined analysis of mouse- and
human-selective inhibitors, chimeric ATGL proteins, and homology models.

11.02.2022
New Paper Series on Teraryl-based alpha-Helix Mimetics
In this series of three back-to-back papers, we report about a complete library of building blocks featuring the side chains of all amino acids relevant for protein-protein interactions (PPIs). Via sequential Pd cross-coupling, these building blocks can be connected to form teraryl-based alpha-helix mimetics as inhibitors of PPIs. The papers are referred to as Parts 3-5 as they follow up on our two previous publications in this field.
Parts 3 and 4 describe core fragments with two leaving groups of differentiated reactivity (iodide and triflate or iodide and bromide, respectively). Part 5 describes a complementary set of pyridine boronic acid building blocks.
Part 3: https://doi.org/10.1002/ejoc.202101278

19.10.2021
New paper on total synthesis of Trabectidin degradation product ETM-204
In our newest publication, we describe the first total synthesis of ETM-204, which is a metabolite of the chemotherapeutic agent Trabectidin (Yondelis®). Key step of the synthesis is a modified Pomeranz–Fritsch cyclization to assemble the isoquinoline ring. The paper was published in Monatshefte für Chemie.
https://link.springer.com/article/10.1007/s00706-021-02844-1

27.07.2021
New paper on transition-state based inhibitors of DPP3
In this study, published in Chemistry – A European Journal, we disclose the design, synthesis and biophysical characterization of peptidomimetic inhibitors of dipeptidyl peptidase III (DPP3). The inhibition of this metalloprotease is based on the entropy-driven release of water from the closing binding cleft as confirmed by ITC measurement and X-ray crystallography.
https://chemistry-europe.onlinelibrary.wiley.com/doi/epdf/10.1002/chem.202102204

11.12.2020
Review on water-soluble phosphorus ligands
Hydrophilic phosphorus ligands are crucial for transition metal-catalyzed reactions in water or biphasic solvents. In our latest review, published in Advanced Synthesis & Catalysis, we summarize the progress in the synthesis of novel water-soluble phosphines over the past decade. Additionally, we describe recent applications of transition metal catalysis in aqueous solvents based on hydrophilic P-donor ligands.
https://onlinelibrary.wiley.com/doi/10.1002/adsc.202001278

02.09.2020
Review on ABPP of Oxidoreductases
This minireview summarizes state of the art approaches for the activity-based protein profiling (ABPP) of oxidoreductases and discusses the scope and limitations of established methodologies.
https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/cbic.202000542

15.07.2020
New paper on SAR studies of ATGL inhibitors
In our latest publication in Bioorganic & Medicinal Chemistry, we disclose structure–activity relationship (SAR) studies of small molecule inhibitors of murine adipose triglyceride lipase (mATGL) that resulted in the development of Atglistatin as powerful tool compound.
https://www.sciencedirect.com/science/article/pii/S0968089620304405

04.05.2020
New paper on stereoselective synthesis of thiol-containing 1,2-aminoalcohols
In this contribution published in Tetrahedron, we report a synthetic protocol for the stereoselective preparation of highly functionalized aminohydroxythiols. The methodology is based on a SmI2/LiBr-mediated reductive coupling of Ellman N-sulfinylimines with aldehydes.
https://www.sciencedirect.com/science/article/pii/S0040402020303926

19.03.2020
New publication on alpha-helix mimetics
In this study a highly modular synthesis of quateraryl alpha-helix mimetics as inhibitors for protein-protein interactions (PPIs) is presented. The combination of an electroorganic as well as a Pd-catalyzed cross-coupling provides efficient access to quaterarylic systems. This work was published in Catalysts.
https://www.mdpi.com/2073-4344/10/3/340

06.03.2020
Congratulations to Anna Migglautsch
Anna Migglautsch just defended her PhD thesis on “Development of inhibitors of human adipose triglyceride lipase (hATGL)”. We want to thank her for the last years in which she became an essential member of the Institute and congratulate on doing an amazing job at her defense!

06.01.2020
Press release on drug development
The Austrian Research Promotion Agency (FFG) just published a press release on the development of a drug candidate for diabetes treatment. This joint research project between the Institute of Molecular Biosciences (University of Graz) and the Institute of Organic Chemistry (TU Graz) focuses on the synthesis of small molecule inhibitors of the adipolytic enzyme ATGL.
https://www.ffg.at/sites/default/files/allgemeine_downloads/strukturprogramme/RSA/RSA_Studios_A3_Atglistatin.pdf

07.11.2019
New study on highly selective Pd-catalyzed S-allylation
In our latest publication we disclose a palladium-catalyzed protocol for the highly chemo- as well as regioselective S-allylation of thiols. This transformation developed at the Institute of Organic Chemistry offers several advantages over established reaction for the synthesis of thioethers. The paper was published in Advanced Synthesis & Catalysis and highlighted as “very important publication”!
https://onlinelibrary.wiley.com/doi/full/10.1002/adsc.201901250

11.10.2019
Press release on new method for the synthesis of lipidated proteins
TU Graz just published a press release on the development of a new method for the site-selective lipidation of proteins. This palladium-mediated reaction was developed at the Institute of Organic Chemistry (TU Graz) in collaboration with the Institute of Biological Chemistry (University of Vienna) and recently published in the Journal of American Chemical Society.
https://www.tugraz.at/en/tu-graz/services/news-stories/media-service/singleview/article/neues-verfahren-zur-schnelleren-und-einfacheren-herstellung-lipidierter-proteine0/
