Sascha Kleiber, Andreas Böhm and Susanne Lux
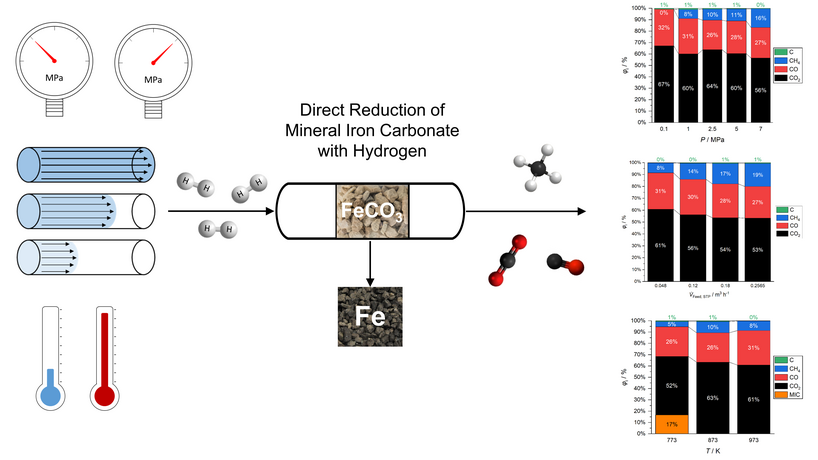
Direct reduction of mineral iron carbonate with hydrogen enables an over 60% reduction of process-related CO2 emissions in iron production, compared to the conventional process route of roasting and reduction with coke. Due to the carbonaceous nature of the ore, the release of carbon-based gaseous products during reduction is inevitable. However, precise adaption of the process parameters temperature and pressure give access to a tailor-made product gas consisting of CO and methane in addition to released CO2; without compromising the product quality of the reduced iron ore.
In the present work, the effect of elevated reaction pressure (0.1–7 MPa) was investigated for different temperatures (773–973 K) and feed gas flow rates (0.0480−0.2565 m3 h−1), and discussed in terms of reaction time, conversion of mineral iron carbonate, degree of metallization, and product gas composition. Elevated reaction pressure increases the degree of metallization of the reduced ore and the methane mole fraction in the product gas. The degree of metallization also increases with increasing temperature. Compared to direct reduction at ambient pressure, the degree of metallization at 2.5 MPa is significantly increased at lower temperatures. With increasing temperature, this difference becomes less evident. With an increasing feed gas flow rate, the methane mole fraction in the product gas increases.
Chemical Engineering Journal, 2024